Abstract
Background
The bone marrow microenvironment (BMEV) regulates the highly regenerative hematopoietic system. However, there are a limited number of BMEV-derived molecules with a definitive role in maintaining hematopoietic stem and progenitor cells (HSPCs).
Extracellular vesicles (EVs) encapsulate bioactive molecules, and may modify the physiology of their target cells. In hematopoiesis, EVs derived from culture-expanded mesenchymal cells can rescue irradiation damage, expand human umbilical cord blood cells and support HSPCs in vitro . However, in vivo evidence of EV function is lacking. We therefore sought to investigate the role of EVs in the interaction between the BMEV and the hematopoietic system and took advantage of existing mice bearing genetic reporters of key mesenchymal cell types.
Results
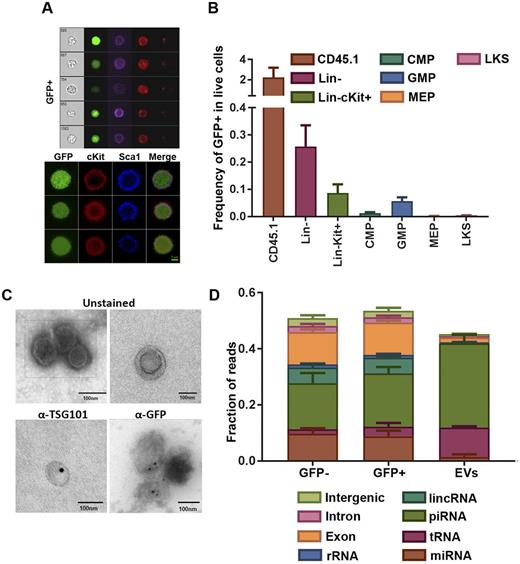
While analyzing the bone marrow (BM) of different mesenchymal cell-GFP reporter mice, we unexpectedly found CD45+ GFP+ cells. These were confirmed as single cells with intracellular GFP as demonstrated by imaging flow cytometry and confocal microscopy (Fig. 1A). Moreover, their hematopoietic identity was confirmed by their ability to form myeloid colonies in methylcellulose.
Transplanted CD45.1 BM into Osteocalcin-Topaz (Ocn-Topaz) and Collagen1-GFP (Col1-GFP) mice that label osteoblasts, as well as Nestin-GFP (Nes-GFP) that labels mesenchymal stem cells demonstrated that donor cells are comparably labeled with GFP in Ocn-Topaz and Col1-GFP (2.2%) but at a much lower frequency (0.05%) in Nes-GFP. We therefore decided to proceed with the Ocn-Topaz model to investigate the role of osteoblast derived EVs in hematopoietic communication.
Within the lineage negative compartment, the frequency of GFP+ cells increased with maturation. The highest frequency found in GMPs (0.06% of live cells were GFP+), followed by CMPs (0.01%), MEPs (0.002%) and LKS (0.004%) (Fig. 1B). Of particular interest, Lin- GFP+ cells formed ~5 fold more colonies as compared to their GFP- counterparts. However, transplantation assays demonstrated that the GFP+ cells possessed a decreased ability for long term reconstitution.
Given the molecular weight of GFP, we hypothesized that EVs were the basis for transfer. Transmission electron microscopy coupled with immunogold staining revealed microvesicular structures of ~100 nm in size that contained GFP and that were labeled with the exosome marker TSG101 (Fig. 1C). Western blotting and flow cytometry detected labeling with exosome markers CD81 and CD9.
Heparin sulfate proteoglycans (HSPGs) have been implicated in the biogenesis and uptake of EVs. Osteoblast-specific disruption of HSPGs by the knock out of the glycosyl transferase EXT1 resulted in a (40%) drop in the frequency of GFP+ cells in the GMP compartment. These findings demonstrate the EV-dependent transfer of GFP from osteoblasts to BM hematopoietic cells, and confirm GFP as a marker for the isolation and characterization of EV target cells.
Exosomes from the BM of Ocn-Topaz mice in addition to GFP+ and GFP- GMPs were isolated for small RNA sequencing. In parallel, GMP populations were collected for mRNA sequencing. Global analysis of small RNA libraries from EVs and GMPs demonstrated that piRNAs was the most abundant species in both EVs (30%) and GMPs (18%). Surprisingly, EVs had low miRNA content (1.4%) compared to GMPs (9.2%) (Fig. 1D). When comparing GFP+ GMPs to GFP- ones, 6 miRNAs (mir-143, mir-122, mir-423-5p, mir-451, mir-206, mir-146b*) showed at least 100% increase in the GFP+GMPs. Predicted targets of mir-143, mir-206, mir-146 emerged as enriched sets when comparing gene expression of GFP+ and GFP- GMPs.
In contrast, tRNAs was the most enriched species in EVs (10.5%) when compared to GMPs (2.5%) (Fig. 1D) and interestingly, GFP+ GMPs had higher content of tRNA when compared to GFP- (3.3% vs 1.7%) respectively. Given the role of tRNAs in translation and the emerging role of tRNA fragments (tRFs) in translation regulation and stress signaling, it was of interest to see translation and ribosome genesis among the top enriched gene sets when comparing GFP+ and GFP- GMPs.
In conclusion, we present evidence for the in vivo transfer of bioactive EVs from osteoblasts to BM progenitor populations, and that this transfer alters hematopoietic cell function and gene expression. Moreover, we identify piRNAs and tRNAs as the most enriched species of small RNAs within BM derived EVs.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.